Publication Summary
Highly multiplexed imaging of single cells using a high-throughput cyclic immunofluorescence method
Jia-Ren Lin1, Mohammad Fallahi-Sichani2, and Peter K. Sorger1
1 HMS LINCS Center, Laboratory of Systems Pharmacology, Harvard Medical School, Boston, MA; 2 Department of Systems Biology, Harvard Medical School, Boston, MA
Nat Commun (2015) 6, Article number: 8390
doi:10.1038/ncomms9390 / PMID:26399630 / PMCID:PMC4587398
Synopsis
There is an emerging need in numerous fields of biological research is to increase the multiplicity of single-cell measurements. Multiplexed single-cell techniques have the potential to reveal important interdependencies between a cell’s local environment and its differentiation status, signal transduction pathway activity, and morphological phenotypes that are not evident when each feature is measured independently. To this end, we developed a robust, multiplexed immunofluorescence imaging method, named Cyclic Immunofluorescence (CycIF), using public domain chemistry and existing instruments that enables low-cost, high-dimensionality imaging assays at the single-cell level.
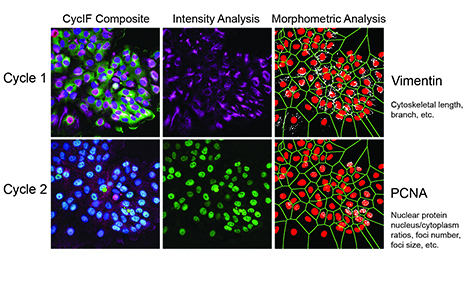
Figure 1. Two rounds of CycIF were performed, yielding the following fluorescent signals: Cycle 1: blue = Hoechst, green = Foxo3a-Venus, red = NLS-mCherry, purple = Vimentin; Cycle 2: blue = Hoechst, green = PCNA, red = p21, purple = Actin). Raw Vimentin and PCNA images first were used for intensity-based analysis. The same images then were binarized and different filters applied (sharpen, skeletonized, maximum, etc.) to extract texture features (length, branches, enrichment, and clusters).
Key Findings
- CycIF is a novel, robust and inexpensive method for performing high-dimensionality fluorescence microscopy using standard instrumentation and reagents.
- We describe three variants of the CycIF protocol that can be applied combinatorially to a single sample to achieve multiple rounds of live-cell fluorescence imaging of reporter-expressing and dye-labeled cells, of direct immunofluorescence imaging using fluor-conjugated antibodies, and of indirect immunofluorescence imaging using secondary antibodies.
- The rich data collected using CycIF are amenable to state-of-the-art, high-dimensionality analysis tools developed for CyTOF, including ViSNE and Wanderlust, that allow investigation of complex associations and interdependencies between observed features and phenotypes.
Abstract
Single-cell analysis reveals aspects of cellular physiology not evident from population-based studies, particularly in the case of highly multiplexed methods such as mass cytometry (CyTOF) able to correlate the levels of multiple signaling, differentiation and cell fate markers. Immunofluorescence (IF) microscopy adds information on cell morphology and the microenvironment that are not obtained using flow-based techniques, but the multiplicity of conventional IF is limited. This has motivated development of imaging methods that require specialized instrumentation, exotic reagents or proprietary protocols that are difficult to reproduce in most laboratories. Here we report a public-domain method for achieving high multiplicity single-cell IF using cyclic immunofluorescence (CycIF), a simple and versatile procedure in which four-color staining alternates with chemical inactivation of fluorophores to progressively build a multichannel image. Because CycIF uses standard reagents and instrumentation and is no more expensive than conventional IF, it is suitable for high-throughput assays and screening applications.
Explore the data and associated resources
We encourage readers to explore the data and protocols described in this study, and we welcome your feedback as we develop the CycIF method further. To promote independent assessment of the method and the results produced by its use, we provide access through an associated project exploration website to the CycIF protocol, to a discussion group where we welcome users to submit questions and suggestions, and to much of the image data and calculated signal intensity data for the figures in this paper so that users can analyze them independently. An associated dataset, which includes image and signal intensity data from a complete, representative 2-cycle CycIF experiment, also is available in the HMS LINCS Database through the link in the table below.
Available data and software
| Data | All raw and registered images, coordinate data for all regions of interest, and calculated metrics for each individual well for a representative, 2-cycle CycIF (HMS Dataset #20236). | Details | Download (.xls) |
| Software | Complete set of image processing scripts for registration and analysis of CycIF data | Download (.zip) |
Funding Sources
NIH LINCS grant U54 HL127365 and a Merck Fellowship of the Life Sciences Research Foundation to MF-S.
Related References
1. Lin, J.R., Fallahi-Sichani, M., Chen, J.Y., and Sorger, P.K. (2016) Cyclic Immunofluorescence (CycIF), a highly multiplexed method for single-cell imaging. Curr Protoc Chem Biol. 8(4):251-264. doi:10.1002/cpch.14 PMID:27925668 PMCID:PMC5233430