Publication Summary
Neutral Loss Is a Very Common Occurrence in Phosphotyrosine-Containing Peptides Labeled with Isobaric Tags
1 Department of Cell Biology, Harvard Medical School, Boston, MA; 2 Laboratory of Systems Pharmacology, Harvard Medical School, Boston, MA; 3 Cell Signaling Technology, Inc., Danvers, MA
J Proteome Res (2017) 16(2):1069-1076.
doi:10.1021/acs.jproteome.6b00487 / PMID:27978624
Synopsis
Mass spectrometry-based methods that enable relative peptide quantification have the potential to provide significant insights into biological mechanisms, but a number of technical challenges currently limit our ability to obtain accurate quantification across samples. After unexpectedly discovering significant neutral loss from phosphotyrosine (pY)-containing peptides in multiplexed pY peptide quantification assays involving isobaric labeling, we investigated this phenomenon quantitatively using a library of 2000 pY peptides and high resolution accurate mass data. By characterizing trends in neutral loss and immonium ion formation during processing of peptide samples with and without isobaric labeling, a set of features was identified that can inform instrument method development and supplement site localization algorithms for the analysis of isobarically labelled peptides, thereby potentially leading to methods to extract more meaningful information from multiplexed phosphotyrosine enrichment studies.
Key Findings
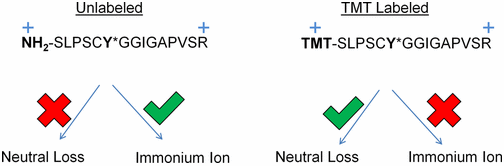
- Using a library of 2000 phosphotyrosine peptides and high resolution accurate mass data, we identified several features of neutral loss in the presence or absence of isobaric labeling that can guide the development of improved methods for accurate detection of all labeled peptides.
- First, phosphotyrosine neutral loss is not a rare event after isobaric tagging.
- Second, neutral loss from isobarically tagged peptides is inversely proportional to proton mobility and does not occur during electron transfer dissociation.
- Third, replacement of a primary amine for a tertiary amine during TMT labeling contributes to neutral loss by increasing gas-phase proton affinity and lowering proton mobility of a TMT-labeled peptide relative to an unlabeled peptide of the same sequence and charge state.
- Fourth, immonium ion formation is inversely proportional to neutral loss formation.
- Knowing when and where to look for HPO3 loss and immonium ion formation can inform instrument method development and supplement site localization algorithms, enabling recovery of peptides with low site localization scores and aiding follow-up experiments, e.g., site mutation and site-specific antibody creation.
Abstract
While developing a multiplexed phosphotyrosine peptide quantification assay, an unexpected observation was made: significant neutral loss from phosphotyrosine (pY) containing peptides. Using a 2000-member peptide library, we sought to systematically investigate this observation by comparing unlabeled peptides with the two highest-plex isobaric tags (iTRAQ8 and TMT10) across CID, HCD, and ETD fragmentation using high resolution high mass accuracy Orbitrap instrumentation. We found pY peptide neutral loss behavior was consistent with reduced proton mobility, and does not occur during ETD. The site of protonation at the peptide N-terminus changes from a primary to a tertiary amine as a result of TMT labeling which would increase the gas phase basicity and reduce proton mobility at this site. This change in fragmentation behavior has implications during instrument method development and interpretation of MS/MS spectra, and therefore ensuing follow-up studies. We show how sites not localized to tyrosine by search and site localization algorithms can be confidently reassigned to tyrosine using neutral loss and phosphotyrosine immonium ions. We believe these findings will be of general interest to those studying pY signal transduction using isobaric tags.
Funding Sources
NIH grants P50 GM107618 (R.A.E., A.R.E., S.P.G.), U54 HL127365 (R.A.E., A.R.E., S.P.G.), and GM67945 (S.P.G.)