Publication Summary
Torin2 exploits replication and checkpoint vulnerabilities to cause death of PI3K-activated triple-negative breast cancer cells.
Sameer S. Chopra1,3,5, Anne Jenney1,3, Adam Palmer1,3, Mario Niepel1,3, Mirra Chung1,3, Caitlin Mills1,3, Sindhu Carmen Sivakumaren2,5, Qingsong Liu2,5, Jia-Yun Chen1, Clarence Yapp1,3, John M. Asara4, Nathanael S. Gray2,5 and Peter K. Sorger1,3,6,*
1Laboratory for Systems Pharmacology, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115, USA
2Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115, USA
3Harvard Ludwig Center, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115, USA
4Division of Signal Transduction, Beth Israel Deaconess Medical Center, Harvard Medical School, 200 Longwood Avenue, Boston, MA 02115, USA
5Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA 02215, USA
6Lead contact
*Correspondence: Peter_Sorger [at] hms.harvard.edu
Cell Syst. 10(1):66-81.e11.
doi:10.1016/j.cels.2019.11.001 PMID:31812693
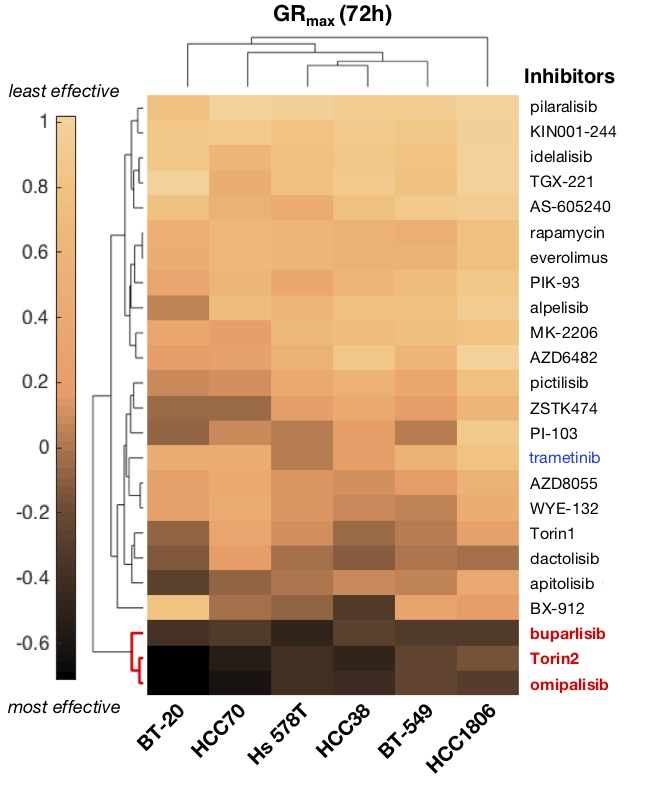
Figure 1b. Clustergram heatmap of mean GRmax values for 24 drugs in six TNBC cell lines; N=3 experiments. Dark shading denotes GRmax<0 (i.e., cytotoxicity). Red, highly effective drugs; blue, MAPK inhibitor.
Synopsis
New therapies are urgently needed for triple-negative breast cancer (TNBC), an aggressive subtype of breast cancer that is incompletely responsive to standard-of-care chemotherapy. Molecular profiling studies show that TNBCs have high PI3K/AKT/mTOR signaling activity, providing a rationale for possible use of PI3K pathway inhibitors. However, the optimal strategy for targeting PI3K pathway dysregulation in TNBC is unknown. Chopra et al. systematically analyzed responses of TNBC cells to a compendium of mechanistically diverse PI3K/AKT/mTOR inhibitors. They found that the preclinical drug Torin2 and a collection of related chemical analogs that inhibit both mTOR and other PI3K-like kinases including ATR and DNA-PK are more potent and cytotoxic than most clinical-grade PI3K pathway inhibitors. Torin2-like molecules may thus represent a new pharmacological strategy for targeting TNBC.
Key Findings
- In contrast to most clinical-grade PI3K pathway drugs, Torin2 induces apoptosis and has strong anti-proliferative effects in TNBC cells.
- A chemical genetic approach showed that the high activity of Torin2 in TNBC results from combined inhibition of mTOR and other PIKKs including ATR, ATM, and DNA-PK.
- Unlike other PI3K pathway drugs, Torin2 causes death of TNBC cells by replication and/or mitotic catastrophe.
- The unique polyselectivity of Torin2 exploits both PI3K-pathway activation and tumor cell replication stress and/or checkpoint dysfunction.
Abstract
Frequent mutation of PI3K/AKT/mTOR signaling pathway genes in human cancers has stimulated large investments in targeted drugs but clinical successes are rare. As a result, many cancers with high PI3K activity, such as triple negative breast cancer (TNBC), are treated primarily with chemotherapy. By systematically analyzing responses of TNBC cells to a diverse collection of PI3K pathway inhibitors, we find that one drug, Torin2, is unusually effective because it inhibits both mTOR and PI3K-like kinases (PIKKs). In contrast to mTOR-selective inhibitors, Torin2 exploits dependencies on several kinases for S-phase progression and cell-cycle checkpoints, thereby causing accumulation of single-stranded DNA and death by replication catastrophe or mitotic failure. Thus, Torin2 and its chemical analogs represent a mechanistically distinct class of PI3K pathway inhibitors that are uniquely cytotoxic to TNBC cells. This insight could be translated therapeutically by further developing Torin2 analogs or combinations of existing mTOR and PIKK inhibitors.
Datasets and Associated Metadata and Code
Data analyses available via https://github.com/datarail/datarail. An introduction to GR metrics can be found at http://www.grcalculator.org. The following data are available for download.
| Figure | Dataset | HMS LINCS ID | Synapse ID |
|---|---|---|---|
| 1B, S1D | Evaluation of the sensitivities of six triple-negative breast cancer (TNBC) cell lines to 23 different PI3K/AKT/mTOR inhibitors or to a MEK inhibitor (trametinib). Dataset 1 of 2: GR values. | 20364 | |
| 1B, S1D | Evaluation of the sensitivities of six triple-negative breast cancer (TNBC) cell lines to 23 different PI3K/AKT/mTOR inhibitors or to a MEK inhibitor (trametinib). Dataset 2 of 2: GR metrics. | 20365 | |
| 5C | Evaluation of the sensitivity of two triple-negative breast cancer cell lines (HCC1806, HCC70) to a collection of dual mTOR and PI3K-like kinase (PIKK) inhibitors. Dataset 1 of 2: GR values. | 20366 | |
| 5C | Evaluation of the sensitivity of two triple-negative breast cancer cell lines (HCC1806, HCC70) to a collection of dual mTOR and PI3K-like kinase (PIKK) inhibitors. Dataset 2 of 2: GR metrics. | 20367 | |
| 2F | Targeted metabolomics profiling data (i.e., peak intensity measurements) after acute exposure of BT-549 cells to DMSO or rapamycin, AZD8055 or Torin2 at two doses (100nM, 1000nM) for 6 hours. | 20368 | |
| 1C, S1E | Breast Cancer Profiling Project, Drug Sensitivity phase I: Fixed-cell GR measures of 35 breast cell lines to 34 small molecule perturbagens from library plate I. Dataset 1 of 2: Normalized growth rate inhibition values | 20343 | |
| 1C, S1E | Breast Cancer Profiling Project, Drug Sensitivity phase I: Fixed-cell GR measures of 35 breast cell lines to 34 small molecule perturbagens from library plate I. Dataset 2 of 2: Calculated dose response metrics | 20344 |
Funding Sources
This work was funded by NIH/NCI grants HL127365 (to P.K.S. and N.S.G.) and CA225088 (to P.K.S. and S.S.C.), the DFCI Leadership Council (S.S.C.), Conquer Cancer Foundation of ASCO (S.C.), and T32 CA 9172-4 (S.S.C.). Mass spectrometry was supported in part by CA120964 (J.M.A.) and CA006516 (J.M.A.).